We at the CVR were recently visited by retrovirologist Dr Kate Bishop (not that one) from the Francis Crick Institute in the centre of London. Like our own Dr Sam Wilson (not that one), Kate works on restriction factors and retroviruses. After our customary podcast was eaten by some terrifying space monkeys, we’ve instead discussed some of Kate’s work in this blog post.
Few viruses have had such a potent impact on the world as the human immunodeficiency virus (HIV), which has led to the death of tens of millions of people, left sufferers with a lifelong infection that cannot be cured, and has had huge economic impacts on countries across the world. Wouldn’t it be great if HIV could be stopped?
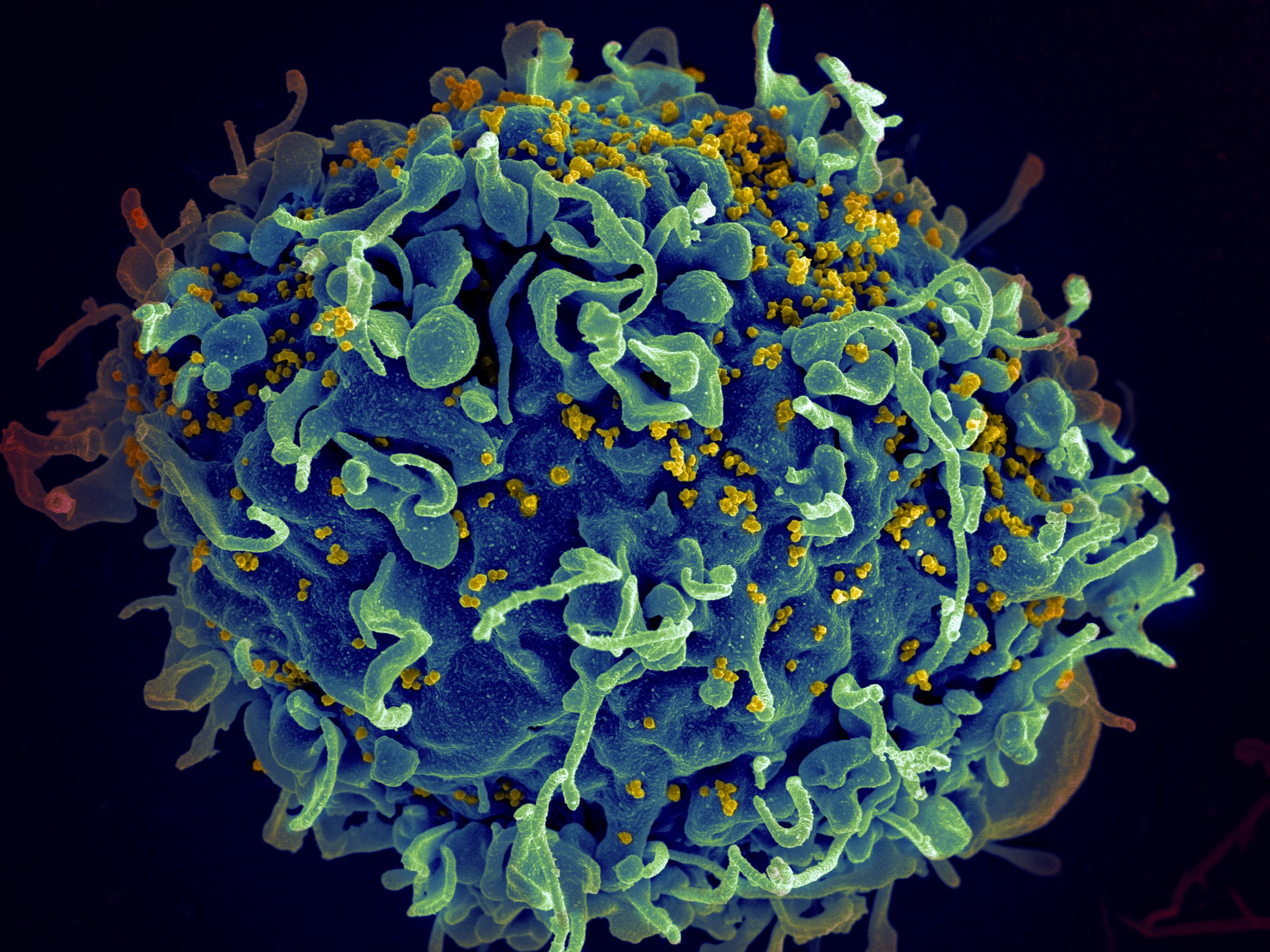
Currently, HIV is treated with antiviral drugs (called antiretrovirals here because HIV is part of the retrovirus family), which are remarkably effective at controlling the infection and if they are taken properly, life expectancy for an individual with HIV will be the same as for an individual without. However, these drugs treat the infection but cannot cure it, making treatment very expensive and life-long. In the absence of a vaccine we therefore need drugs that will completely cure the infection.
Developing such treatments requires us to understand more about how the virus works and interacts with our body. Many scientists across the world – and here at the CVR – are actively researching in this area, including Dr Kate Bishop. Scientists, including Kate, think that kinds of molecules known as a ‘restriction factors’ may be the key to controlling HIV more effectively.
Restriction factors blocking infection
Viruses are parasites of our cells and therefore infection usually comes at a cost to us and our cells. To cause an infection, a virus has to overcome a formidable gauntlet of host defences. These host defences have evolved over millions of years in our bodies as a way a stop the bad effects of viral infection. Viruses have to contend with physical barriers like skin, chemical barriers like stomach acid, complement proteins that blow holes in foreign-looking membranes, innate immune cells like macrophages and adaptive immune cells like T and B cells. In addition to systemic defences, viruses have to contend with a group of genes and their proteins that work by blocking virus infection at the level of the initial infected cell.
These antiviral proteins are termed restriction factors (they restrict the virus) and are incredibly important at stopping viruses from making us sick (compare these with ‘dependency factors’ that viruses use in our cells to help them replicate).
There are many examples of restriction factors, which can stop an array of viruses from causing infection. For retroviruses like HIV, we have evolved APOBEC proteins, which mutate viral genomes beyond the point of usefulness; tetherin, which literally tethers virus particles to cell membranes; and TRIM5α, which physically stops the genome from escaping the virus coat (also known as the capsid). If you want to learn more about these proteins then check out this great review from Mike Malim and Paul Bieniasz.
These proteins are constantly produced by our cells so that they are ready to stop infection from the moment a virus first enters a cell. The cell also increases their production after signalling from interferon, a protein produced by infected cells that tells their neighbours to start ramping up their defences.
For an infection to take hold, viruses must counteract the cell’s restriction factors. One way that viruses achieve this is by producing molecules called accessory proteins. Understanding how restriction factors work may allow us to develop new ways to control or cure viral diseases. This is a focus of the Bishop lab in London, whose recent work has focused on one critical HIV restriction factor, SAMHD1 (or sterile alpha motif and HD domain-containing protein 1, if you’ve got a lot of time to type).
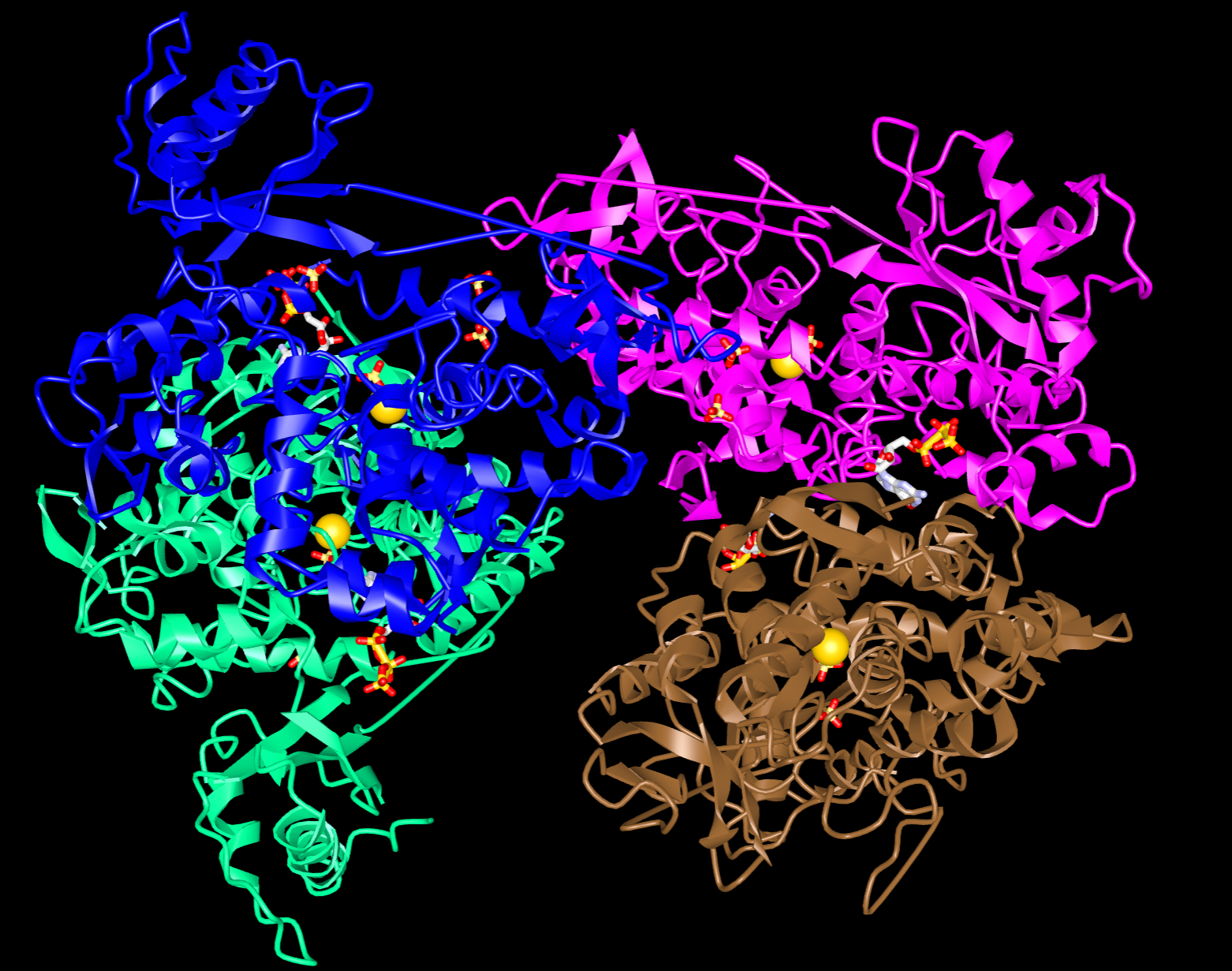
Tell me more about SAMHD1
Kate came to the CVR on June 21st and gave a seminar about her and her lab’s work on understanding SAMHD1. Here she explained that the protein was initially identified by comparing different strains of HIV. Since 1998 it has been known that myeloid cells, which include macrophages and dendritic cells, are difficult to infect with the most common strain of HIV, HIV-1 (that came from chimpanzees).
However, a less common strain, HIV-2 (that came from sooty mangabeys), is able to infect these cells. The difference is due to HIV-2 possessing an accessory protein called Vpx, which in 2011 was shown to target SAMHD1. In 2014 Kate’s group helped to show how this targeting takes place: Vpx acts as an adaptor between SAMHD1 and the ubiquitin system, which marks proteins for degradation.
Current understanding is that SAMHD1 blocks HIV infection by shrinking the pool of dNTPs, the building blocks of DNA that HIV requires to copy its RNA genome into DNA. Although this doesn’t seem to be a specific antiviral response; it’s just a housekeeping process that the cell needs itself but just so happens to inhibit HIV replication.
In 2015, the Bishop lab published a paper detailing the structure of SAMHD1 and found two key sites: an active site to bind and degrade all dNTPs, and a regulatory site which binds only dGTP (or similar G-based nucleotides). dGTP binding of the regulatory site promotes organisation into a tetramer (four molecules stuck together), which is required for enzyme activation. The cell can also switch tetramer formation off by adding a phosphate group to SAMHD1, and switch formation on by removing the phosphate group. The presence of these switches raises the prospect of using drugs to regulate the activity of SAMHD1, which could affect its antiviral capacity.
The Bishop lab’s most recent work demonstrates that modulating SAMHD1 levels within cells can affect the effectiveness of commonly used HIV drugs. Furthermore, SAMHD1 can be modulated by several nucleoside analogues, a class of drugs which resemble dNTPs but prevent DNA synthesis. These drugs are already used to treat HIV and several herpesvirus infections.
The paper also suggests that many drugs used to treat herpesvirus infections would actually be effective against HIV in differentiated cells with very low dNTP concentrations, which make up a substantial proportion of HIV-infected cells. This raises the tantalising prospect of using these drugs, which are cheaper and more readily available than current HIV treatments, as cost-effective treatments for HIV in resource-poor settings.
Want to be like Kate?

Kate had some additional time to talk to the Contagious Thinking team about her background, training and advice to young scientists, explaining that she had always been interested in science from a very early age but she solidified this passion during her undergraduate degree where she was fortunate enough to work in Sweden and the USA as part of sandwich placements during her undergraduate degree. It was here during these trips that she got exposed to the scientific life and critically, worked in two very different environments, which she felt gave her a rounded understanding of real-world science (something that she recommends to other young scientists).
Kate began working on retroviruses due to the number and quality of research tools that had been developed and was fascinated by the understanding that virology could bring to cell biology. Kate did her undergraduate at the University of Bath (including placements at the Karolinska Institute in Stockholm and the Chiron Corporation in San Francisco) and her PhD with Jonathan Stoye at the National Institute for Medical Research before postdoctoral training with Michael Malim at King’s College London. She has recently received tenure at the Francis Crick Institute in London. Kate has been incredibly supportive of public engagement with science and hopes to continue this into the future.
Dr Kate Bishop is a researcher at the Francis Crick Institute. You can find her profile page, including a list of her publication here. To find out more about the work on HIV and restriction factors carried out at the CVR, you can check out our retrovirology podcast or blog post. Restriction factors aren’t limited to HIV, scientists at the CVR are developing new tools to identify and characterise restriction factors from multiple host species that protect against a number of important human and animal pathogens.

