The last week of June saw Europe’s first confirmed human case of West Nile fever of the 2015 season, in Sofia, Bulgaria. West Nile fever, which is caused by West Nile virus (WNV) can be a serious disease, sometimes resulting in brain inflammation, paralysis and even death. While not unprecedented in the region, Europe has seen a dramatic rise in West Nile fever cases in the last decade and cases have been increasing. Europe will likely see a couple of hundred cases by the end of the year. This surge in disease cases is not unique to Europe; North America already sees thousands of cases of West Nile fever a year despite not having any cases before 1999.
What is alarming is that infections like WNV, spread by insects and ticks, are predicted to increase their distribution in the future, due to a number of factors that scientists are trying to understand i.e urbanisation, globalisation (one example being the spread of mosquito larvae with the tyre trade) and possibly climate change. There is little we can do to their expansion and spread of disease, that is unless we unravel the secrets of how they work, which is something the MRC-University of Glasgow Centre for Virus Research is investing a lot of time and effort into investigating.

Arboviruses are a diverse and complex group of viruses
When you think of a virus you likely conjure up images of those that cause measles, chickenpox, cold or mumps. These viruses all share one major thing in common, that they are all spread to humans, from humans, by humans. Yet some viruses are transmitted in a wholly different way and are spread by arthropods, such as mosquitoes, midges, sandflies or ticks. These ‘arboviruses’ (arthropod-borne viruses) use arthropods as ‘vectors’ to help them transmit. Whereas some viruses spread just in arthropods, arboviruses have evolved to exploit their blood-feeding hosts and are able to infect vertebrates, like us, and also replicate in them, which plays an important role in their life-cycle.
Arboviruses are ubiquitous in nature. They are found worldwide and can infect all kinds of vertebrates, including humans and livestock. Importantly, many of these viruses cause disease and even death in their vertebrate hosts. WNV, dengue, chikungunya, yellow fever, Japanese encephalitis, Rift Valley fever, tick-borne encephalitis, Crimean-Congo haemorrhagic fever, bluetongue, Schmallenberg, African horse sickness and African Swine fever viruses (to name a few!) are all classed as arboviruses and they all can cause disease.
“Arboviruses are ubiquitous in nature, are found worldwide and can infect all kinds of vertebrates, including humans and our livestock.”

The term arbovirus betrays a massive amount of biological and viral diversity and there are many different kinds of arboviruses. While nearly exclusively RNA viruses, arboviruses include some very distinct kinds of viruses. Of note, the African swine fever virus – spread by ticks – is the only arboviruses with a DNA genome. While even within the ‘RNA arboviruses’, you have positive sense and negative sense and segmented and non-segmented and then those segmented ones may have 3 or 8 segments… Yet in spite of this genetic and biological diversity, the distinction between arboviruses and non-arboviruses remains an important one because in sharing similar vector biology their may be similar ways to stop them through the control of mutual vectors. This is important because arboviruses can cause some pretty nasty illnesses.
Arboviruses exert a significant public health burden
While arboviruses do not generally cause recognisable disease in their arthropod vectors they often do in vertebrates, in particular warm-blooded vertebrates like primates like us and livestock. Vertebrate arbovirus infections are generally acute like flu rather than chronic, like HIV and HCV, although most remain sub-clinical. While disease is not uncommon, when present, symptoms can include fever, arthralgia, encephalitis and haemorrhage depending on host and virus. In general the case fatality rate is in the low single percentages yet because so many individuals may become infected, this amounts to a very significant total. As an example, the WHO states that for dengue, “…390 million dengue infections [occur] per year (95% credible interval 284–528 million), of which 96 million (67–136 million) manifest clinically (with any severity of disease).” Dengue’s case-fatality rate is less than 1% and mostly amongst children.
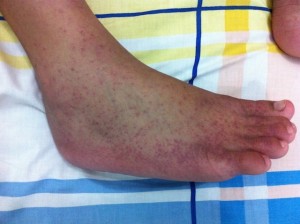
Being viruses, antibiotics are not effective and no cures exist currently for any arbovirus. No specific treatments are licensed against them and when disease presents, supportive care is recommended alongside treating the symptoms. Control of arbovirus infections is largely concentrated on prevention, through the use of vector control, physical defence against bites and vaccination. Vaccines are licensed against yellow fever, Japanese encephalitis and tick-borne encephalitis viruses, which are very safe, effective and affordable (at least for those in developed countries). Vaccines are currently in development for WNV, dengue and chikungunya viruses but they have not completed testing and reached the public yet. However, now an intensive research focus is aimed at manipulating the arthropod vector biology to control arboviruses as an alternative way to prevent these infections.
“Being viruses, antibiotics are not effective and no cures exist.”
Arthropods as efficient virus spreaders
Arthropods are an ancient (>500 million years old) and amazingly diverse group (phylum) of invertebrate animals with unique and fascinating biology. They include four living – and one extinct – kinds (sub-phyla): the Hexapoda (insects, such as mosquitoes and flies like drosophila), Chelicerata (spiders and ticks), Crustacea (crabs and lobsters) and Myriapoda (millipedes), alongside the extinct Trilobitomorpha (trilobites). Contesting to their evolutionary success, arthropods are the most diverse group of animals living (mainly due to the insects) and have conquered most ecological niches, including hot and dry; land, sea and air. Additionally they may be small or big, and can have incredibly large population sizes.
One amazing thing about arthropods is the evolution of ‘haematophagy’ or blood-feeding (in the insects, mosquitoes/midges and some flies, as well as ticks). While other animals feed on blood (leeches, nematodes, vampire bats and oxpecker birds) and many other diseases (parasitic, bacterial and viral) are spread by these non-arthropod blood-feeding animals, the arthropods are the most prolific. The precise reason for their importance as disease vectors is unknown but likely lie in a number of unique adaptations, in particular their blood-feeding capacity and their ability to fly- but also possibly in their immune responses, physiology and the ability of viruses and parasites (and some bacteria) to adapt to these.
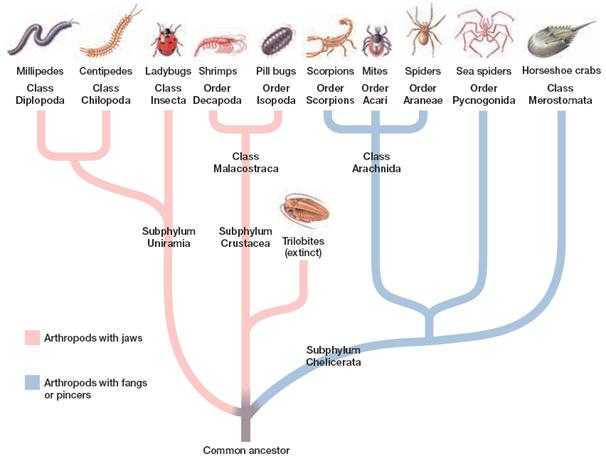
“…arthropods are the most diverse group of animals living (mainly due to the insects) and have conquered most ecological niches…”
Despite all this biological diversity, only a small number of arthropods are vectors and only a small number of viruses are arboviruses (although we are finding more and more as each year goes on). Even of all the arbovirus vectors, the mosquitoes are considered the most important vectors and have been termed the ‘most lethal’ of all animals, acting as vectors for dengue, chikungunya, yellow fever, Japanese encephalitis and West Nile viruses.
Altering vector ecology and biology for arbovirus control
Understanding the biology and ecology of arboviruses may hold the key to their control. This is a very complex process but can be best understood in three components: virus, vector and vertebrate. Most arboviruses are maintained in transmission cycles that involve replication in both hosts. This vertebrate host may be humans but can also be other primate species, birds or even reptiles. Some of these hosts can transmit virus on to new arthropods but some are dead ends because the virus does not grow so well, as in WNV in humans. Those hosts that the virus grows well in and do not get sick, may act as reservoirs for the arbovirus allowing the virus to survive (like birds for WNV). In other cases, such as dengue (originally a sylvatic virus), human-only transmission cycles take place.
Arbovirus distribution is thus dictated by the presence of vector and/or reservoir species, if one is necessary. One of the major factors controlling vector distribution is climate, in particular temperature and rainfall for some mosquito species. Climate change may therefore play a role in how these diseases and their vectors will cause problems in the future. However, chance effects also play a role, such as the slave trade did in bringing yellow fever, traditionally a disease in Sub-Saharan Africa, to the Americas.
“Arbovirus distribution is thus dictated by the presence of vector and/or reservoir species, if one is necessary.”
While it is difficult for us to manipulate our climate easily, there exist now targeted ways to affect vector biology, including insecticides, physical barriers and even disruption of vector breeding. New biological control means are beginning to emerge, including generating genetically-modified mosquitoes or using symbiotic bacteria species to alter vector biology to resist arbovirus infection.
What we are doing to help
Arboviruses can cause significant disease and even death in humans and other animals. This worldwide morbidity and mortality is likely to change and in some areas, increase. Despite large arbovirus diversity, they share a unique but common biology: as viruses that exploit arthropods to infect and cause disease in their vertebrate hosts. Thus understanding arbovirus-vector-host interactions may lead to control strategies, which are so desperately needed.
In recognition of this, the CVR has a broad, collaborative programme on arboviruses as part of its ‘Emerging & zoonotic viral infections’ theme. In particular, the CVR investigates their molecular virology, virus-host interactions, vaccine development and vector biology across the different programme member labs of Alain Kohl, Esther Schnettler, Emilie Pondeville, Margus Varjak, Richard Elliott and Massimo Palmarini. This strategy allows us to be prepared for future, often unpredictable, problems with arboviruses, but also work on ongoing issues.
Our response to Schmallenberg virus, which emerged unexpectedly in Europe, is a case in point; within a short time we pooled resources to study the virus and vector, and developed attenuated viruses that could serve as vaccine candidates.
*********************************************************************************************************************
Of note, the MRC-University of Glasgow Centre for Virus Research will host the 2015 International Meeting on Arboviruses and their Vectors (#IMAV15), as a Society for General Microbiology-sponsored ‘focus meeting’. #IMAV15 will be held on Monday 7th and Tuesday 8th September 2015 in Glasgow and confirmed speakers include Marco Vignuzzi (Pasteur Institute, France), Michael Diamond (Washington University in St Louis, USA), Lisa Fong Poh Ng (A*Star, Singapore) and Zoe Curtis (Oxitec, UK). You can still register and submit an abstract here.
*********************************************************************************************************************
By Connor Bamford (@cggbamford) with help from the fellow blog editors (Joanna and Veronica), Naomi and Alain.

