
As the International Meeting on Arboviruses and their Vectors, kicks off today in Glasgow with the Society for General Microbiology (#IMAV15), we’d like to present to you the fifth and final in a series of posts about arboviruses, their vertebrate hosts and their arthropod vectors. This post, written by Dr Alain Kohl ,CVR Arthropod-borne infections programme leader along with Dr Esther Schnettler, CVR senior investigator scientist, focuses on arbovirus vectors, their biology and how by understanding how they interact with arboviruses and their vertebrate hosts, we can make safe and effective control strategies for some of the world’s worst diseases.
The arthropod vector i.e. the blood-feeding insect (for examples mosquitoes, midges) or arachnid (in case of tick-borne viruses) plays a central – and essential – role in the ecology of arboviruses. They are not passive transmitters of viruses, many arthropods are actively infected by arboviruses, where they can replicate.
“by focusing on arthropods we should be able to interrupt this infectious cycle.”
Understanding the mechanisms and dynamics of arthropod infection by arboviruses is of great importance because targeting the arthropod itself, instead of the diverse viruses and vertebrate hosts they infect, may be a powerful means to control arbovirus disease in humans and livestock. But this means having to study their own biology, something that is fundamentally different from mammals, like us, and other vertebrates that are infected by arboviruses.
Arthropods – an essential role in arbovirus ecology
Arboviruses usually infect arthropods after taking a blood meal from an infected vertebrate, and then replicate in the tissues of the arthropod. Replication within the arthropod – first in the epithelial cells that line their gut and then other tissues – allows the virus to vastly increase its numbers. This means that there is more chance of starting a new infection but allows access to the all important salivary glands, the major secretory organs.
Transmission can then occur by inoculation of this saliva containing arboviruses at the next blood meal. This is the main reason why by focusing on arthropods we should be able to interrupt this transmission cycle.
Arthropods fight back
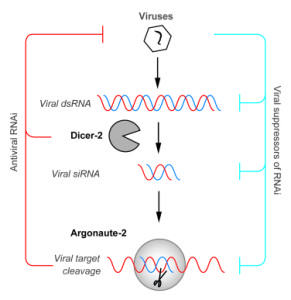
Viruses as parasites exert a burden on their hosts and accordingly, hosts have evolved means to detect, respond to – and reduce that burden. Arthropods are no different. Unlike vertebrates, which have the interferon system along with their inhibitory ‘interferon stimulating genes’, ( as well as their adaptive immune response) arthropods to rely other diverse pathways, such as RNAi, Toll, immune-deficiency and the JAK-STAT pathways.
One of the most powerful antiviral pathways that arthropods have is the RNAi pathway, which is functional and inhibits viruses in ticks, mosquitos and midges. In essence, RNAi detects and remembers viral RNA within the host cells and captures this RNA to target nucleases to virus genomes and degrade them, thus restricting infection.
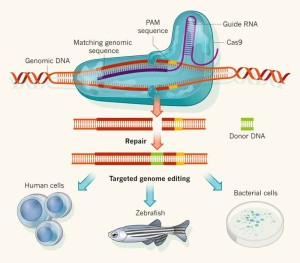
“We can even alter the genome of vector species using efficient and specific techniques, such as transposon or CRISPR-Cas9-mediated genome editing.”
Manipulating the vector
Generally speaking, vectors remain a key intervention target as few vaccines against arboviruses exist and are licensed, and that includes the important human pathogen dengue. These vector-based control measures can involve eradication programmes with insecticides, habitat control etc.
Modern biology also tries to understand how arboviruses and vectors interact, for example by identifying which host factors and immune responses control viruses in the arthropod. This can help efforts to design novel strategies for example based on trait selection or genetic modification. We can even think about altering the genome of vector species using efficient and specific techniques, such as transposon or CRISPR-Cas9-mediated genome editing.
Other non-genetic means of vector manipulation include exploiting vector-infecting ‘endosymbiont’ bacteria species, such as Wolbachia. Infection of arthropod cells with these bacteria can inhibit virus infection.
However, each arbovirus-vector combination comes with its own characteristics that make it challenging to develop an all-encompassing control strategy. Some arboviruses such as the human-infecting dengue and chikungunya viruses are closely associated with specific mosquitoes, such as Aedes aegypti and Aedes albopictus. Others such as Rift Valley fever virus are more promiscuous and can infect and be transmitted by many species. Others again have more complex endemic and epidemic cycles that involve different vectors. This should drive us to understand the biology of each of these different vector species.
Keeping a close eye on vectors
While Western Europe has been relatively sheltered from human arboviruses, the midge borne blue tongue and Schmallenberg viruses have had considerable impact on agriculture and animal health. Other parts of Europe are known for cases of West Nile and tick-borne encephalitis viruses, while Crimean-Congo hemorrhagic fever is also present in some regions.
Importantly though the now well established presence of Aedes mosquitoes in parts of Europe and import of viruses such as dengue and chikungunya has led to small outbreaks of these exotic viruses in the South of the continent. This shows that we need to keep a close eye on vectors and continue our efforts to control these.
*********************************************************************************************************************
The MRC-University of Glasgow Centre for Virus Research will host the 2015 International Meeting on Arboviruses and their Vectors (#IMAV15), as a Society for General Microbiology-sponsored ‘focus meeting’. #IMAV15 will be held on Monday 7th and Tuesday 8th September 2015 in Glasgow and confirmed speakers include Marco Vignuzzi (Pasteur Institute, France), Michael Diamond (Washington University in St Louis, USA), Lisa Fong Poh Ng (A*Star, Singapore) and Zoe Curtis (Oxitec, UK).
The CVR has a broad, collaborative programme on arboviruses as part of its ‘Emerging & zoonotic viral infections’ theme. In particular, the CVR investigates their molecular virology, virus-host interactions, vaccine development and vector biology across the different programme member labs of Alain Kohl, Esther Schnettler, Emilie Pondeville, Margus Varjak, Richard Elliott and Massimo Palmarini. This strategy allows us to be prepared for future, often unpredictable, problems with arboviruses, but also work on ongoing issues.

